Whether you're diabetic or not, it is important that you
keep your immune system strong to protect you against most diseases and
illness, including the flu and the common cold.
Your immune system protects your body against disease by
identifying and killing pathogens and tumor cells. It detects a wide variety of
agents, from viruses to parasitic worms, and needs to distinguish them from
your own healthy cells and tissues in order to function properly. Detection is
complicated as pathogens can evolve rapidly, and adapt to avoid the immune
system and allow the pathogens to successfully infect our bodies.
When you catch a cold or the flu; or, when you develop a
disease such as diabetes or cancer, the primary reason is due to inflammation and a weakened immune system that is unable to defend your body against
the invading pathogens, viruses, fungi, and parasites; and the other health issues
such as insulin resistance, high blood pressure, nutrient deficiencies, poor
diet, and toxins.
Consequently, two of the most critical steps in being
able to successfully prevent or defeat
any illness or disease are to reduce the inflammation
and strengthen the immune system. But, first,
let's take a look at how the immune system works.
Immune System: 3 Lines of Defense
The
immune system is a collection of special cells, tissues and molecules that
protects the body from numerous pathogenic microbes and toxins, utilizing 3
lines of defense:
1.
Physical and Chemical Barriers (Innate Immunity)
2.
Nonspecific Resistance (Innate Immunity)
3.
Specific Resistance (Acquired or Adaptive Immunity)
1st Line
of Defense: Physical and Chemical Barriers
Physical
Barriers include: the skin; mucous membranes; hair within the nose; cilia which
lines the upper respiratory tract; urine which flushes microbes out of the
urethra; defecation and vomiting which expel microorganisms.
Chemical
Barriers include: lysozyme, an enzyme produced in tears, perspiration, and
saliva can break down cell walls and thus acts as an antibiotic (kills
bacteria); stomach gastric juice which destroys bacteria and most toxins; sebum
(unsaturated fatty acids) provides a protective film on the skin and inhibits
growth.
2nd Line
of Defense: Nonspecific Resistance (Innate Immunity)
The
second line of defense is nonspecific resistance that destroys invaders in a
generalized way without targeting specific individuals. White blood cells
(called phagocytes) ingest and destroy all microbes that pass into body
tissues.
In
addition, there is an inflammatory response in the localized tissue where the
pathogen invaded the body or where the tissue was damaged due to a cut or
wound. Inflammation brings more white blood cells to the site where the
microbes have invaded. The inflammatory response produces swelling, redness,
heat, pain and fever. Fever inhibits bacterial growth and increases the rate of
tissue repair during an infection.
3rd Line
of Defense: Specific Resistance (Acquired Immunity)
The third line of defense is specific resistance. This
system relies on antibodies, which are produced by specific immune cells
(called B cells) in response to the antigens on the surface of the invading
pathogens.
When an antigen is detected by a macrophage, this causes
the T cells to become activated. The activation of T cells by a specific
antigen is called cell-mediated immunity. The body contains millions of
different T cells, each able to respond to one specific antigen.
The T cells secrete interleukin 2, which causes the
proliferation of certain cytotoxic T cells and B cells. T cells stimulate B
cells to divide, forming plasma cells that are able to produce antibodies and
memory B cells.
If the same antigen enters the body later, the memory B
cells divide to make more plasma cells and memory cells that can protect
against future attacks by the same antigen.
When the T cells activate (stimulate) the B cells to
divide into plasma cells, this is called antibody-mediated immunity.
Antibodies (also called immunoglobulins) are Y-shaped
proteins that circulate through the blood stream and bind to specific antigens,
thereby attacking microbes. The antibodies are transported through the blood
and the lymph to the pathogen invasion site.
The body contains millions of different B cells, each
able to respond to one specific antigen.
Antibodies bind to an antigen, preventing its normal
function or making it easier for phagocytic cells to ingest them; or, they
activate a complement protein that kills the pathogen or signals other white
blood cells; or they binds to the surface of macrophages to further facilitate
phagocytosis.
Immune System Components
The major components of the immune system include the
following:
Thymus: is located between your
breast bone and your heart and is responsible for producing thymosin, which
helps to activate T cells. As we get
older, this organ shrinks over 80% and produces less thymosin and may be one of the reasons why
our immune system weakens and we become more susceptible to certain diseases.
Spleen: filters the blood
looking for foreign cells (the spleen is also looking for old red blood cells
in need of replacement). A person missing their spleen gets sick much more
often than someone with a spleen.
Lymph system: includes the tissues
and organs, including the bone marrow, spleen, thymus, and lymph nodes, that
produce and store cells that fight infection and disease. The channels that
carry lymph are also part of this system.
Lymph is a clear-like liquid that bathes the cells with
water and nutrients. Lymph is blood plasma -- the liquid that makes up blood
minus the red and white cells. Think about it -- each cell does not have its
own private blood vessel feeding it, yet it has to get food, water, and oxygen
to survive. Blood transfers these materials to the lymph through the capillary
walls, and lymph carries it to the cells.
The cells also produce proteins and waste products and
the lymph absorbs these products and carries them away. Any random bacteria
that enter the body also find their way into this inter-cell fluid. One job of
the lymph system is to drain and filter these fluids to detect and remove the
bacteria. Small lymph vessels collect the liquid and move it toward larger
vessels so that the fluid finally arrives at the lymph nodes for processing.
Bone marrow: produces new blood
cells, both red and white including B cells. In the case of red blood cells the
cells are fully formed in the marrow and then enter the bloodstream. In the
case of some white blood cells, the cells mature elsewhere. The marrow produces
all blood cells from stem cells. They are called "stem cells" because
they can branch off and become many different types of cells - they are
precursors to different cell types. Stem cells change into actual, specific
types of white blood cells.
White blood cells: also called leukocytes,
are probably the most important part of your immune system. These cells work
together to destroy bacteria and viruses. The different types of white blood
cells include: Neutrophils, Eosinophils, Basophils, Monocytes, Lymphocytes, B cells,
T cells, Helper T cells, Suppressor T cells, Killer T cells, Granulocytes,
Plasma cells, Phagocytes, Dendritic cells, Natural Killer cells, and
Macrophages.
Antibodies: (also referred to as
immunoglobulins) are produced by white blood B cells. They are Y-shaped
proteins that each respond to a specific antigen
(bacteria, virus or toxin). Antibodies come in five classes: Immunoglobulin A (IgE),
Immunoglobulin D (IgE), Immunoglobulin E (IgE), Immunoglobulin G (IgG), and Immunoglobulin
M (IBM).
Antigen: The surface of every
cell is covered with molecules that give it a unique set of characteristics.
These molecules are called antigens. Antigens are generally fragments of
protein or carbohydrate molecules. There are millions of different antigens and
each one has a unique shape that can be recognized by white blood cells. The
white blood cells then produce antibodies to match the shape of the antigens.
Some antigens (e.g. associated with bacteria, viruses,
pollen, etc.) stimulate an immune response by a white blood (B) cell to
generate antibodies specific to that antigen that matches the shape of the
antigen.
Now the antibody can bind to that specific antigen to
make it easier for other white blood cells to engulf or attack the bacteria or
virus who brings the antigen with them.
The antigens on the surface of bacteria, viruses and
other pathogenic cells are different from those on the surface of your own
cells. This enables your immune system to distinguish pathogens from cells that
are part of your body. Antigens are also found in foods like peanuts and on the
surface of foreign materials like pollen, pet hairs and house dust where they
can be responsible for triggering an allergy, hay-fever or asthma attacks.
Lymphokines: are several hormones
generated by components of the immune system. It is also known that certain
hormones in the body suppress the immune system. Steroids and corticosteroids
(components of adrenaline) suppress the immune system.
Tymosin (thought to be produced
by the thymus) is a hormone that encourages lymphocyte production.
Interleukins are another type of hormone
generated by white blood cells. For example, Interleukin-1 is produced by
macrophages after they eat a foreign cell. IL-1 has an interesting side-effect
- when it reaches the hypothalamus it produces fever and fatigue. The raised
temperature of a fever is known to kill some bacteria.
Lymphokines are a subset of cytokines that are produced
by lymphocytes. They are protein mediators typically produced by T cells to
direct the immune system response by signaling between its cells.
Lymphokines
have many roles, including the attraction of other immune cells, including
macrophages and other lymphocytes, to an infected site and their subsequent
activation to prepare them to mount an immune response. Circulating lymphocytes
can detect a very small concentration of lymphokine and then move up the
concentration gradient towards where the immune response is required.
Lymphokines aid B cells to produce antibodies.
Important lymphokines secreted by the T helper cell
include: interleukin 2, 3, 4, 5, 6; granulocyte-macrophage colony-stimulating
factor; and interferon-gamma.
Cytokines: are small peptides
that act as signaling systems within the body. Because they facilitate
communication between the innate and adaptive immune systems, cytokines are a
key factor in fighting infection and maintaining homeostasis.
Cytokines include chemokines, interferons, interleukins,
lymphokines, tumor necrosis factor. Cytokines are produced by a broad range of
cells, including immune cells like macrophages, B lymphocytes, T lymphocytes
and mast cells, as well as endothelial cells, fibroblasts, and various stromal
cells.
Proinflammatory cytokines such as interleukin 1 (IL-1)
and tumor necrosis factor alpha (TNF-alpha) are released defensively in
response to infection and trauma. Anti-inflammatory cytokines such as
transforming growth factor beta (TGF-beta) and IL-10 oppose the action of the
proinflammatory cytokines and promote healing.
Some cytokines are chemical switches that turn certain
immune cell types on and off. One cytokine, interleukin 2 (IL-2), triggers the
immune system to produce T cells. IL-2’s immunity-boosting properties have
traditionally made it a promising treatment for several illnesses.
Elevated plasma levels of proinflammatory cytokines are
biomarkers of inflammation and/or disease. An imbalance between the activity of
proinflammatory and anti-inflammatory cytokines is believed to affect disease
onset, course, and duration.
Anti-inflammatory cytokines is a general term for those
immunoregulatory cytokines that counteract various aspects of inflammation, for
example cell activation or the production of pro-inflammatory cytokines, and
thus contribute to the control of the magnitude of the inflammatory responses.
These mediators act mainly by the inhibition of the production of
pro-inflammatory cytokines.
The major
anti-inflammatory cytokines are IL4, IL10, and IL13, and IL35. Other
anti-inflammatory mediators include IL16, IFN-alpha, TGF-beta, IL1ra, G-CSF, as
well as soluble receptors for TNF or IL6.
Tonsils: are lymphoepithelial
tissues facing into the aerodigestive tract. These tissues are the immune
system's first line of defense against ingested or inhaled foreign pathogens.
The fundamental immunological roles of tonsils aren't yet understood.
Lymph nodes are distributed widely
throughout areas of the body, including the armpit and stomach, and linked by
lymphatic vessels. Lymph nodes are garrisons of B, T and other immune cells.
Lymph nodes act as filters or traps for foreign particles and are important in
the proper functioning of the immune system. They are packed tightly with the
white blood cells, called lymphocytes and macrophages.
Skin: is one of the most
important parts of the body because it interfaces with the environment, and is
the first line of defense from external factors, acting as an anatomical
barrier from pathogens and damage between the internal and external environment
in bodily defense. Langerhans cells in the skin are part of the adaptive immune
system.
Liver: has a wide range of
functions, including immunological effects—the reticuloendothelial system of
the liver contains many immunologically active cells, acting as a
"sieve" for antigens carried to it via the portal system.
They also store information on these non-self substances
to be able to react faster the next time. The large bowel also contains
bacteria that belong to the body, called gut flora. These good bacteria in the
large bowel make it difficult for other pathogens to settle and to enter the
body.
Innate and Adaptive Immunity
The protection provided by the immune system is divided
into two types of reactions: reactions of innate immunity and reactions of
adaptive or acquired immunity.
The innate immune
system consists of cells and proteins that are always present and ready to
mobilize and fight microbes at the site of infection. The main components of
the innate immune system are 1) physical epithelial barriers; 2) phagocytic
leukocytes (neutrophils, eosinophils, basophils); 3) monocytes (which develop
into macrophages); 4) dendritic cells; 5) a special type of lymphocyte called
natural killer (NK) cells; and, 6) circulating plasma proteins. Other
participants in innate immunity include the complement system and cytokines
such as interleukin 2 (IL-2).
Innate immune cells express genetically encoded
receptors, called Toll-like receptors (TLRs), which recognize general danger-
or pathogen-associated patterns. Collectively, these receptors can broadly
recognize viruses, bacteria, fungi, and even non-infectious problems. However,
they cannot distinguish between specific strains of bacteria or viruses.
There are numerous types of innate immune cells with
specialized functions. They include neutrophils, eosinophils, basophils, mast
cells, monocytes, dendritic cells, and macrophages.
Their main feature is the ability to respond quickly and
broadly when a problem arises, typically leading to inflammation. Innate immune
cells also are important for activating adaptive immunity. Innate cells are
critical for host defense, and disorders in innate cell function may cause
chronic susceptibility to infection.
The adaptive (or
acquired) immune system is called into action against pathogens that are
able to evade or overcome innate immune defenses. Components of the adaptive
immune system are normally silent; however, when activated, these components
“adapt” to the presence of infectious agents by activating, proliferating, and
creating potent mechanisms for neutralizing or eliminating the microbes. There
are two types of adaptive immune responses: 1) humoral
immunity, mediated by antibodies produced by B lymphocytes; and, 2) cell-mediated immunity, mediated by T lymphocytes.
The adaptive immune response is more complex than the
innate. The antigen first must be processed and recognized. Once an antigen has
been recognized, the adaptive immune system creates an army of immune cells
specifically designed to attack that antigen. Adaptive immunity also includes a
"memory" that makes future responses against a specific antigen more
efficient.
Each receptor recognizes an antigen, which is simply any molecule that may bind to a BCR or TCR. Antigens are derived from a variety of sources including pathogens, host cells, and allergens. Antigens are typically processed by innate immune cells and presented to adaptive cells in the lymph nodes.
If a B or T cell has a receptor that recognizes an antigen from a pathogen and also receives cues from innate cells that something is wrong, the B or T cell will activate, divide, and disperse to address the problem. B cells make antibodies, which neutralize pathogens, rendering them harmless. T cells carry out multiple functions, including killing infected cells and activating or recruiting other immune cells.
Certain T cells (Helper T) help activate B cells to secrete antibodies and macrophages to destroy ingested microbes. They also help activate other T cells called cytotoxic T cells to kill infected target cells. As dramatically demonstrated in AIDS patients, without Helper T cells we cannot defend ourselves even against many microbes that are normally harmless.
However, Helper T cells themselves can only function when activated to become effector cells. They are activated on the surface of antigen-presenting cells (APC), which mature during the innate immune responses triggered by an infection.
The
innate responses also dictate what kind of effector cell a Helper T cell will
develop into and thereby determine the nature of the adaptive immune response
elicited.
The
adaptive immune response has a system of checks and balances to prevent
unnecessary activation that could cause damage to the host. If a B or T cell is
auto-reactive, meaning its receptor recognizes antigens from the body's own
cells, the cell will be deleted. Also, if a B or T cell does not receive
signals from innate cells, it will not be optimally activated.
Immune
memory is a feature of the adaptive immune response. After B or T cells are activated,
they expand rapidly. As the problem resolves, cells stop dividing and are
retained in the body as memory cells. The next time this same pathogen enters
the body, a memory cell is already poised to react and can clear away the
pathogen before it establishes itself.
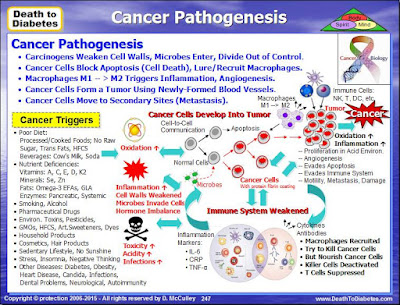
A
further aspect of the adaptive immune system worth mentioning is its role in
monitoring body cells to check that they aren't infected by viruses or
bacteria, for instance, or in order to make sure that they haven't become
cancerous. Cancer occurs when certain body cells 'go wrong' and start dividing
in an uncontrolled way.
The Major Cells of the Immune System
The key tissues and organs involved with the immune
system include the lymph nodes, spleen, tonsils, bone marrow, thymus, and lymphatic
tissue. The key immune cells are white blood cells (or leukocytes). The (3)
major categories of white blood cells are: granulocytes,
lymphocytes and monocytes.
Granulocytes are characterized by the presence of granules in
their cytoplasm which contain digestive enzymes that kill various types of
bacteria and parasites. Granulocytes are also called polymorphonuclear
leukocytes (PMN, PML, or PMNL) because of the varying shapes of the nucleus,
which is usually lobed into three segments. The principal types of granulocytes
are neutrophils, eosinophils, basophils, and mast cells.
Lymphocytes come in three major types:
B-lymphocytes (or
B cells), T-lymphocytes(or T cells) and natural killer (NK) cells.
Lymphocytes start out in the bone marrow and either stay
there and mature into B cells, or they leave for the thymus gland, where they
mature into T cells.
B cells produce antibodies in
the humoral immune response and are like the body's military intelligence
system, seeking out their targets and sending defenses to lock onto them. With
the help of T cells, B cells make special Y-shaped protein antibodies, which stick
to antigens on the surface of bacteria, stopping them in their tracks, creating
clumps that alert your body to the presence of intruders.
Plasma cells, also called plasma B
cells, secrete large volumes of antibodies.
Memory B cells are important in
generating an accelerated and more robust antibody-mediated immune response in
the case of re-infection (also known as a secondary immune response).
Regulatory B
cells (Bregs) participates in immunomodulations and in suppression of immune
responses. via production of anti-inflammatory cytokine interleukin 10 (IL-10).
T cells recognize and kill
virus-infected cells directly. Some help B cells to make antibodies, which
circulate and bind to antigens. Others send chemical instructions (cytokines)
to the rest of the immune system. Types of T cells include Helper T (Th),
Memory T (Tm), Cytotoxic T (Tc), Suppressor T (Treg), and Effector T cells.
Helper T Cells
(Th) help
activate B cells to secrete antibodies and macrophages to destroy ingested
microbes, but they also help activate cytotoxic T cells to kill infected target
cells. Note: In AIDS patients,
without helper T cells we cannot defend ourselves even against many microbes
that are normally harmless.
Memory T Cells
(Tm) are
derived from normal T cells that have learned how to overcome an invader by
‘remembering’ the strategy used to defeat previous infections. At a second
encounter with the invader, memory T cells can reproduce to mount a faster and
stronger immune response than the first time the immune system responded to the
invader.
Cytotoxic T
Cells (Tc)
are lymphocytes that kill invading pathogens including cancer cells, cells that
are infected (particularly with viruses), or cells that are damaged in other
ways. Tc cells kill their targets by programming them to undergo apoptosis. The
elimination of infected cells without the destruction of healthy tissue
requires the cytotoxic mechanisms of CD8 T cells to be both powerful and
accurately targeted.
Suppressor T
Cells (Treg)
suppress the immune response after invading organisms are destroyed by releasing
their own lymphokines to signal all other immune-system participants to cease
their attack.
Effector T
cells
(also called Helper T (Th) cells), are the functional cells for executing
immune functions. Balanced immune responses can only be achieved by proper regulation
of the differentiation and function of Th cells.
Natural killer
(NK)
cells are cytotoxic cells that participate in the innate immune response and
attack in packs by releasing substances that perforate the "skin" of
their victims -- this is death by cell lysis.
Monocytes, which are the largest
of all leukocytes, fight off bacteria, viruses and fungi. Originally formed in
the bone marrow, they are released into the blood and tissues. When certain
germs enter the body, they quickly rush to the site for attack within 8–12
hours.
Monocytes have several functions to help you ward off
diseases and infections. To help you remember what they do, note that each
function begins with the letter 'M': Munch, Mount and Mend.
Munch: Monocytes have the
ability to change into another cell form called macrophages before facing the germs.
In response to inflammation signals, monocytes move quickly to sites of
infection in the tissues and divide/differentiate into macrophages and dendritic cells to elicit an immune
response. They change into macrophages when they move from the bloodstream to
the tissues.
They consume, or munch, on harmful bacteria, fungi and
viruses. Then, enzymes in the monocyte kill and break down the germs into
pieces.
Mount: Monocytes help other
white blood cells identify the type of germs that have invaded the body. After
consuming the germs, the monocytes take parts of those germs, called antigens,
and mount them outside their body like flags. Other white blood cells see the
antigens and make antibodies designed to kill those specific types of germs.
Mend: Monocytes help mend
damaged tissue by stopping the inflammation process. They remove dead cells
from the sites of infection, which repairs wounds. They have also shown to
influence the formation of some organs, like the heart and brain, by helping
the components that hold tissues together.
Macrophages are derived from
monocytes, granulocyte stem cells, or the cell division of pre-existing
macrophages. Macrophages do not have granules but have receptors to detect,
capture and ingest pathogens. Macrophages are found throughout the body in
almost all tissues and organs, just below the surface of the skin and mucous
membranes — any place where a pathogen could get through the first line of
defense. Macrophages cause inflammation through the production of
interleukin-1, interleukin-6, and TNF-alpha. Macrophages are usually only found
in tissue and are rarely seen in blood circulation.
They take up and destroy necrotic cell debris and foreign
material including viruses, bacteria, and tattoo ink. In wound healing,
macrophages take on the role of wound protector by fighting infection and
overseeing the repair process. Macrophages also produce chemical messengers,
called growth factors, which help repair the wound.
When inflammation occurs, monocytes undergo a series of
changes to become macrophages and target cells that need eliminating.
Once engulfed, cellular enzymes inside the macrophage
destroy the ingested particle. Some macrophages act as scavengers, removing
dead cells while others engulf microbes.
Another function of macrophages is to alert the immune
system to microbial invasion. After ingesting a microbe, a macrophage presents
a protein on its cell surface called an antigen, which signals the presence of
the antigen to a corresponding T helper cell.
Macrophages change into foam
cells in the blood vessel walls (endothelium), where they try to
fight atherosclerosis by engulfing excessive cholesterol engulf large amounts
of fatty substances, usually cholesterol. Foam cells are created when the body
sends macrophages to the site of a fatty deposit on the blood vessel walls. The
macrophage wraps around the fatty material in an attempt to destroy it and
becomes filled with lipids (fats). The lipids engulfed by the macrophage give
it a "foamy" appearance.
Foam cells are often found in the fatty streaks and
plaques inside the blood vessel walls. Foam cells do not give off any specific
signs or symptoms, but they are part of the cause of atherosclerosis. Foam cell
development can be slowed, however. Decreasing low density lipoprotein (LDL)
cholesterol and increasing high density lipoprotein (HDL) cholesterol will
remove the lipids that the macrophages engulf to become foam cells.
In addition to the monocytes and macrophages, the other types of white
blood cells include neutrophils, basophils, eosinophils, mast cells
and dendritic cells.
Neutrophils defend against bacterial or fungal infection and other
very small inflammatory processes. They are usually the first responders to
microbial infection; their activity and death in large numbers forms pus.
Basophils are chiefly responsible for allergic reactions and
antigen response by releasing the chemical histamine, which helps to trigger
inflammation, and heparin, which prevents blood from clotting.
Eosinophils primarily deal with
parasitic worm infections. They are also the predominant inflammatory cells in
allergic reactions.
Mast cell is a type of granular
basophil cell in connective tissue that releases heparin, histamine, and
serotonin during inflammation and allergic reactions.
Dendritic
cells
(DCs), which can also develop from monocytes, are an important
antigen-presenting cell (APC) whose main function is to process antigen
material and present it on the cell surface to the T cells in order to activate
the T cells. They act as messengers between the innate and the adaptive immune
systems. Once activated, dendritic cells migrate to the lymph nodes where they
interact with T cells and B cells to initiate and shape the adaptive immune
response.
Note: Antigens are molecules
from pathogens, host cells, and allergens that may be recognized by adaptive
immune cells.
Antigen-presenting
cells
(APCs) like DCs are responsible for processing large molecules into
"readable" fragments (antigens) recognized by adaptive B or T cells
in order to activate them. However, antigens alone cannot activate T cells.
They must be presented with the appropriate major histocompatibility
complex (MHC) molecule "tags" expressed on the APC. MHC
provides a checkpoint and helps immune cells distinguish between self and non-self
cells.
An APC can be any of various cells (as a macrophage or a
B cell) that take up and process an antigen into a form that when displayed at
the cell surface in combination with an MHC molecule is recognized by and
serves to activate a specific helper T cell using their T-cell receptors (TCRs).